Spirometry
LASA filenames:
LASA168
Contact: Natasja van Schoor
Background
Ageing is a process where the physiological integrity progressively decreases, leading to impaired function including decline in lung capacity (1). Spirometry is a simple and reliable test of pulmonary function. It provides information about the airflow and the vital capacity of the lungs. The test can be performed with or without the use of a bronchodilator drug. Spirometry is used to diagnose lung diseases (e.g. Asthma or Chronic Obstructive Pulmonary Disease) (2) and is increasingly recognized as a marker for general health status (3, 4) .
Spirometry in LASA
Spirometry was performed with a Spiro USB Spirometer (Micromedical Ltd, Chatham, Kent, UK). This spirometer is capable of accumulating volume for (minimal) 15 seconds and has an adequate resistance to airflow, based on the ATS/ERS guidelines. It has a resolution of 10 ml volume 0.03 l/s flow and is able to measure volumes of minimal eight liters with an accuracy of at least ±3% of reading or ±0.050 liter (2).
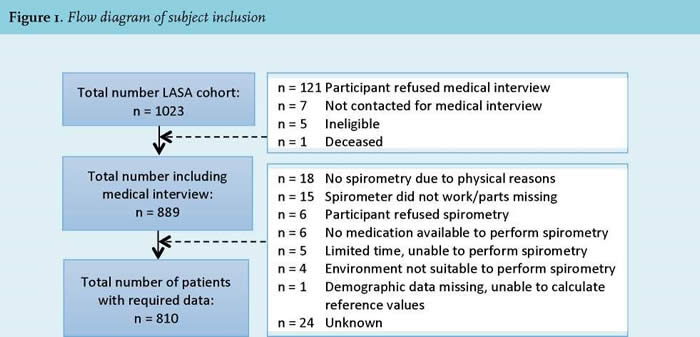
The Micro Medical Spirometer was used in combination with the procedure for spirometry recommended by ATS/ERS (2). Before performing the spirometry, the Spiro USB Spirometer was calibrated with a three litre pump. A correction value between 0.9 and 1.1 was considered reliable. Age, height, and gender were registered to be able to calculate reference values. All participants selected from the LASA 3rd cohort (see Figure 1 for the inclusion procedure underwent a post-bronchodilator test. A dose of 200 mg salbutamol (Airomir autohaler, Teva) was inhaled 10-15 minutes prior to the spirometry. In order to execute the lung function measurement properly, participants received the following instructions; 1) sit straight with both feet on the ground, 2) remove clothing that is (too) tight and loose fitting dentures and 3) enclose the mouthpiece with mouth and lips. After the instructions a nose clip was put on the nose and the participant had to put the mouthpiece of the Spiro USB Spirometer in their mouth. The participant was asked to fully inhale, fully exhale with less than 1 second in between and then fully inhale again. The exhalation had to be performed fast, powerful, smooth, and had to last for at least six seconds. This forced breathing maneuver was repeated until three acceptable maneuvers were performed (up to eight attempts). To meet the requirements of reproducibility, the two highest forced expiratory volume in the first second (FEV1) values and the two highest forced vital capacity (FVC) values were not allowed to differ more than 0.15 liters (150 ml). After performing the test, the best FVC and FEV 1 values (of the three acceptable maneuvers) from the participants were compared with the reference values of people with the same age, height, gender and ethnicity. Subsequently, the Tiffeneau (FEV1/FVC) index was calculated. Results indicate how much a person deviates from the norm-group, based on predicted reference values (5).
Information on variables in spirometry:
- FVC is the maximal volume which can be expired in a exhalation with maximally forced effort from a maximal inspiration.
- FEV1 allso called one-second value, is the maximal volume of air exhaled in the first second of a forced expiration from a position of full expiration.
- Tiffeneau index (FEV1/FVC) indicates the percentage ratio between FEV1 and FVC.
Questionnaires
LAS3B168 (medical interview, in Dutch)
Variable information
LAS3B168
(pdf)
Availability of information per wave ¹
| B | C | D | E | 2B* | F | G | H | 3B* | MB* | I | J | K | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spirometry | - | - | - | - | - | - | - | - | Me | - | - | - | - | |
¹ More information about the LASA data collection waves is available here.
* 2B=baseline second cohort;
3B=baseline third cohort;
MB=migrants: baseline first cohort
Me=data collected in medical interview
Previous use in LASA
Spirometry data were included in a study that aimed to investigate the impact of COPD on health status in Dutch young old adults. This study provides, among other things, basic descriptives about the scores on variables linked to spirometry in the LASA sample.
- Franssen FME, Smid DE, Deeg DJH et al. The physical, mental, and social impact of COPD in a population-based sample: results from the Longitudinal Aging Study Amsterdam. npj Prim Care Resp Med 2018; 28: 30.
- Smid DE, Spruit MA, Deeg DJH, Huisman M, Poppelaars J, Wouters EM, Franssen FME. How to determine an impaired health status in COPD: Results from a population-based study. Neth J Med 2017;75:151-157.
References
- Rutten EP, Gopal P, Wouters EFM, Franssen FME, Hageman GJ, Vanfleteren LE, et al. Various mechanistic pathways representing the ageing process are altered in COPD. Chest. 2015;Epub ahead of print.
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005 Aug;26(2):319-38. PubMed PMID: 16055882. Epub 2005/08/02. eng.
- Hole DJ, Watt GC, Davey-Smith G, Hart CL, Gillis CR, Hawthorne VM. Impaired lung function and mortality risk in men and women: findings from the Renfrew and Paisley prospective population study. BMJ. 1996 Sep 21;313(7059):711-5; discussion 5-6. PubMed PMID: 8819439. Pubmed Central PMCID: 2352103.
- van den Borst B, Gosker HR, Zeegers MP, Schols AM. Pulmonary function in diabetes: a metaanalysis. Chest. 2010 Aug;138(2):393-406. PubMed PMID: 20348195.
- Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, Yernault JC. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J Suppl. 1993;16:5-40.
Other:
https://www.nice.org.uk/guidancemenu/conditions-and-diseases/respiratory-conditions
Date of last update: April, 2020
